How Many Electrons Are in the Outer Shell of Magnesium
So magnesium needs to lose two electrons from its 3s sub-shell to get full outer shell. For the element of MAGNESIUM you already know that the atomic number tells you the number of electrons.
The Number Of Electron Levels In A Magnesium Atom Is At Level
How many electrons are in the outer shell of the magnesium on the periodic table.
. Magnesium needs to lose two electrons to get a full outer shell. VOTE Reply Bogart Marilyn 2. The atom consist of a small but massive nucleus surrounded by a cloud of rapidly moving electrons.
Why Does Magnesium Have 2 Electrons. Its ground state electronic configuration is 1s2 2s2 2p6 3s2. It has two electrons in its outer shell.
Hence electronic configuration of Mg2 is 1s2 2s2 2p6 which is similar to stable electronic configuration of inert gas Ne. A magnesium ion has the same electronic structure as a neon atom Ne. Mg2 is an ion of magnesium that has given up.
It is alkaline earth metal. The electron configuration would be 1s2 2s2 2p6 3s2. When these electrons are lost a magnesium ion Mg 2 is formed.
Mg2 is an ion of magnesium that has given up. Since the atomic number is 12 it has 12 electrons 2 of which are in the outer shell. The atomic number of magnesium is 12.
Mg2 ion is formed by loss of two electrons by Mg atom. Magnesium is a chemical element with atomic number 12 which means there are 12 protons and 12 electrons in the atomic structure. It is electrically neutral with 2 electrons in its outer valence shell.
Magnesium is also a valence electron of 2. Looking at the picture you can see there are two electrons in shell one eight in shell two and two more in shell three. How many electrons does Magnesium have in its outer most shell.
More about the history and places to find magnesium. The nucleus is composed of protons and neutrons. How many electrons are in the outer shell of magnesium in the compound magnesium chloride MgCl 2.
It can either share these two outer electrons or lose them. The electron arrangement of magnesium is 282 meaning it has two outer electrons. It is electrically neutral with 2 electrons in its outer valence shell.
What is the difference in the number of electrons between the magnesium ion and elemental magnesium. The atomic number is the number of electrons in that element. Group of answer choices.
Additionally how many protons neutrons and electrons does mg2 have. Therefore 2 electrons in its outer. The chemical symbol for Magnesium is Mg.
Mg is an atom of the element magnesium. Mg is an atom of the element magnesium. There are 2 electrons in outer shell of Magnesium on periodic table.
That means there are 12 electrons in a magnesium atom. Magnesium Mg Magnesium is in Group 2. The atomic number of magnesium is 12.
Magnesium has a total of 12 electrons 2 in the innermost shell 8 in the second shell and two electrons in its valence shell third shell. Therefore the magnesium atom will have two electrons in the first shell eight in the 2nd orbit and two electrons in the 3rd shell. The electronic configuration of neutral Mg is 1s2 2s2 2p6 3s2 VOTE Reply Chris Beulah 2 years ago Follow A periodic table would be very informative here.
Magnesium has a total of 12 electrons. That is the number of electrons in magnesium is twelve.
How Many Electrons Does Magnesium Have Quora
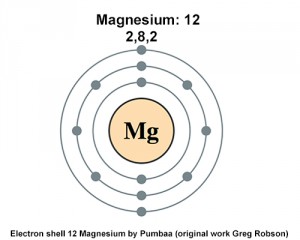
Magnesium Has 12 Protons How Many Electrons Are In Its First Energy Level Socratic
No comments for "How Many Electrons Are in the Outer Shell of Magnesium"
Post a Comment